Abstract
Introduction: Isatuximab (Isa) plus pomalidomide (P) and dexamethasone (d; Isa-Pd) is approved in patients with relapsed/refractory multiple myeloma (RRMM) who have received ≥2 prior therapies, based on the Phase 3 ICARIA-MM study (NCT02990338). Prior to Isa-Pd regulatory approval, Isa was available in France under early access programs (EAPs). In the non-interventional, retrospective IMAGE study, we analyzed the progression-free survival (PFS) and safety among French pts with RRMM treated with Isa-Pd under the EAPs. Here, we report results from the interim subgroup analysis of the IMAGE study based on prior lines of therapy (LOT) and refractory status.
Methods: The IMAGE study was conducted based on medical records of adult patients with RRMM who received ≥1 dose of Isa under the EAPs. The analysis of effectiveness endpoints was restricted to patients with ≥1 year of follow-up after Isa initiation. PFS was defined as the time from start of Isa-Pd to date of disease progression or death. Refractoriness was defined per IMWG criteria. Adverse events (AEs) were not graded for severity. Verbatim terms for AEs were coded using the Medical Dictionary for Regulatory Activities.
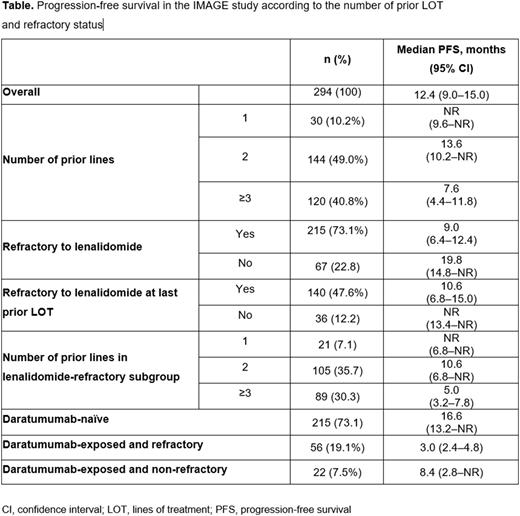
Results: Across 62 study sites in France, 299 patients who had received ≥1 Isa dose under the EAPs from 29 Jul 2019 -1 Sep 2020 were included in the safety population; the effectiveness population included 294 pts who met all inclusion/exclusion criteria. In contrast to ICARIA-MM, the IMAGE study included daratumumab-refractory patients and patients who had received only 1 prior LOT. In the effectiveness population, 10.2%, 49.0%, 40.8% received 1, 2, and ≥3 prior LOT, respectively; 96.3% had lenalidomide; 26.5% had daratumumab; 73.1% were lenalidomide-refractory; and 19.1% were daratumumab-refractory. At a median follow-up of 14.2 months (mo), median PFS (mPFS) of the overall effectiveness population was 12.4 (95% CI 9.0-15.0) mo (Table) with a median 2 prior LOT; this is similar to that reported for daratumumab plus Pd (12.4 mo) in the APOLLO study and for Isa-Pd (11.1 mo) in the ICARIA-MM study, where patients received a median of 2 and 3 prior LOT, respectively. Median PFS was not reached (NR; 95% CI 9.6-NR), 13.6 (95% CI 10.2-NR), and 7.6 (95% CI 4.4-11.8) mo among patients with 1, 2, and ≥3 prior LOT, respectively; 9.0 (95% CI 6.4-12.4) mo in lenalidomide-refractory subgroup; and 10.6 (95% CI 6.8-15.0) mo in lenalidomide-refractory at last prior LOT (Table). Within the lenalidomide-refractory subgroup, mPFS (95% CI) was NR (6.8-NR), 10.6 (6.8-NR), and 5.0 (3.2-7.8) mo with 1, 2, and ≥3 prior LOT, respectively. Among daratumumab-naïve patients, median PFS (95% CI) was 16.6 (13.2-NR) mo. Patients exposed to daratumumab had a median washout period of 6.5 mo between last daratumumab injection and Isa initiation. Patients exposed and refractory to daratumumab had a mPFS of 3.0 (2.4-4.8) mo with a median washout period of 4.0 mo, while patients who were exposed to daratumumab but were not refractory had a mPFS of 8.4 (95% CI 2.8-NR) mo and a median washout period of 9.5 mo. At least 1 AE was reported in 8 (26.7%), 38 (26.4%), and 33 (26.4%) patients in 1, 2, and ≥3 prior LOT subgroups; 60 (27.5%) in lenalidomide-refractory subgroup; and 43 (30.7%) in lenalidomide-refractory at last prior LOT subgroup. AEs led to permanent Isa discontinuation in 3 (2.1%) patients with 2 prior LOT, 1 (0.8%) patient with ≥3 prior LOT, 4 (1.8%) patients in lenalidomide-refractory subgroup, and 3 (2.1%) patients in lenalidomide-refractory at last prior LOT subgroup.
Conclusions: In this subgroup analysis of the IMAGE study based on prior LOT and refractory status, Isa-Pd demonstrated meaningful mPFS with a manageable safety profile at first and second relapse and in the difficult-to-treat lenalidomide-refractory subgroups. Importantly, a high mPFS of 16.6 mo was reported for daratumumab-naïve patients. In the APOLLO and ICARIA-MM trials investigating daratumumab plus Pd and Isa-Pd in a daratumumab-naïve population, mPFS was 12.4 and 11.1 mo respectively. The IMAGE study shows daratumumab-refractory patients do not respond well to Isa-Pd. However, as PFS outcomes were favorable in patients who were exposed to but not daratumumab-refractory, anti-CD38 antibody sequencing in successive therapies may be a good alternative in real-world practice when patients may be exposed to daratumumab but not refractory.
Funding: Sanofi.
Disclosures
Decaux:Takeda: Honoraria; Roche: Honoraria; Gilead: Honoraria; Janssen: Honoraria; BMS: Honoraria; GSK: Honoraria; Sanofi: Honoraria. Lafore:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Iaquinta:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Tekle:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Leleu:BMS: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria; Pfizer: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal